Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Studies of Kinetics and Non-Isothermal Decomposition of Synthesized Novel Organic Terpolymer Resin
Authors: N. C. Das, W. B. Gurnule
DOI Link: https://doi.org/10.22214/ijraset.2024.64546
Certificate: View Certificate
Abstract
The terpolymer resin 2,4-DHP-4-PAF-III was prepared by condensation of 2,4-dihydroxypropiophenone, 4-pyridylamine and formaldehyde in the presence of 2M hydrochloric acid as a catalyst in the temperature range 125 ± 2 0C for 5 hrs in 3:1:5 molar ratios of reactants. Elemental analysis, FT-IR, and 1H-NMR spectral studies have been carried out to confirm the structure of the resin. The thermal degradation behavior of terpolymer was studied using thermogravimetric analysis (TGA) in air atmosphere at a heating rate of 10 0C/min. Sharp-Wentworth and Freeman-Carroll methods were used to calculate thermal activation energy (Ea), entropy change (?S), free energy change (?F), apparent entropy change (S*), frequency factor (Z) and order of reaction (n). Thermal activation energy (Ea) calculated by Sharp-Wentworth (28.81 KJ/mole) method and Freeman-Carroll (29.47 KJ/mole) method are in good agreement.
Introduction
I. INTRODUCTION
Terpolymeric resin has been attracting much attention of materialists since last two decades owing to interesting superior properties that can fulfill the demand of modern society. These include toughness, resilience, low density, high and low melting points, ability to be molded, conductivity, resistance etc [1].Thermal analysis plays a very important role in studying the structure and properties of any material. Thermogravimetric analysis has been widely used to investigate the decomposition characteristics of polymeric matter. Copolymers can be used as high energy material, semiconductors, ion-exchanger, antioxidants, binders, fire proofing agent, optical storage data, molding materials etc. Copolymers were applied in various fields of research as ion-exchangers, high thermal resistance materials, and electrical appliances [2]. Copolymer resins are derived from Melamine, aniline and formaldehyde in hydrochloric acid as catalyst and studied their thermal degradation by Dharkar et. al [3]. Gurnule et.al have studied the thermal degradation and antibacterial study of transition metal complexes derived from 8-hydroxyquinoline-5-sulphonic acid, formaldehyde and guanidine [4]. Belsare and coworkers studied the thermogravimetric analysis of 2, 2’-biphenol based terpolymer resins derived from 2, 2’-biphenol and tetra ethylenepentamine with formaldehyde [5]. Synthesis, characterization and non-isothermal thermogravimetric analysis of cross-linked phenol based copolymer resins was studied by Kalbende et. al [6]. Synthesis and characterization of polymer nanocomposites filled with nanoclay/aerosil on mechanical and thermal properties have been reported by R. Srinivasulu et. al [7]. However, the literature studies have revealed that no resin has been synthesized using the monomers 2 4-dihydroxypropiophenone, 4-pyridylamine with formaldehyde. Thus the present paper deals with thermal analysis of the newly synthesized terpolymer by using 2 4-dihydroxypropiophenone, 4-pyridylamine with formaldehyde. By applying the Sharp-Wentworth and Freeman-Carroll methods. Energy of activation (Ea), thermodynamic parameters viz. Z, ?S, ?F, S*, and order of reaction (n) were determined.
II. MATERIALS AND METHODS
All the chemicals were AR grade and chemically pure grade. 2, 4-Dihydroxypropiophenone, 4-pyridylamine and formaldehyde, DMSO and DMF were procured from market. Double distilled water was used for all experiments.
A. Synthesis of 2, 4-DHP-4-PAF-III Copolymer Resin
The new terpolymer 2, 4-DHP-4-PAF-III was synthesized by condensing 2 4-dihydroxypropiophenone and 4-pyridylamine with formaldehyde in a molar ratio of 3:1:5 in the presence of 2M, 200 ml HCl as a catalyst at 125 ± 2 0 C for 5h, in an oil bath with occasional shaking.
The separated terpolymer was washed with hot water and methanol to remove unreacted starting materials and acid monomers. The properly washed resin was dried, powdered and then extracted with diethyl ether and then with petroleum ether to remove 2, 4-dihydroxypropiophenone-formaldehyde terpolymer which might be present along with 2,4-DHP-4-PAF-III terpolymer. The product was immediately removed from the flask as soon as reaction period was over and then purified. The terpolymer was purified by dissolving in 10% aqueous sodium hydroxide solution, filtered and reprecipitated by gradual drop wise addition of ice cold 1:1 (v/v) concentrated hydrochloric acid / distilled water with constant and rapid stirring to avoid lump formation. The process of reprecipitation was repeated twice. The terpolymer sample 2,4-DHP-4-PAF-III thus obtained was filtered, washed several times with hot water, dried in air, powdered and kept in vacuum desiccator over silica gel. The reaction and suggested structure of 2,4-DHP-4-PAF-III is presented in Figure-1.
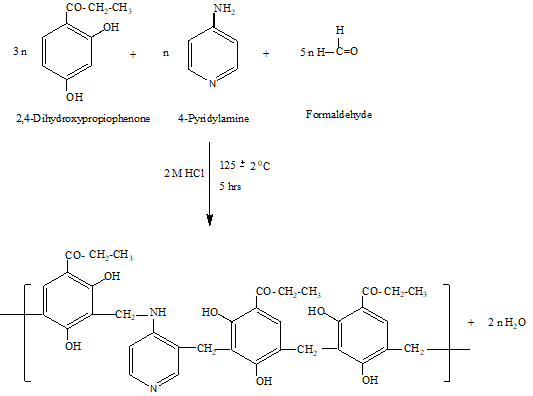
Fig.1: Synthesis of 2,4-DHP-4-PAF-III terpolymer resin
B. Thermogravimetric Analysis
The non-isothermal thermogravimetric analysis method is associated with a change in weight with respect to temperature. Heating is performed under strictly controlled conditions and can reveal changes in structure and other important properties of the material being studied. In TGA, the sample is subjected to conditions increase in temperature at linear rate. The thermogravimetric analysis was performed in air atmosphere with heating rate of 10 0C min-1 using 5-6 mg of samples in platinum crucible from temperature of 40 0C to 800 0C and thermogram was recorded for 2,4-DHP-4-PAF-III at SAIF, Cochin University, Cochin. From the TGA curves, the thermoanalytical data and the decomposition temperatures were determined for different stages. Sharp-Wentworth and Freeman-Carroll method is used to obtain the relative thermal stability of the copolymer.
C. Theoretical Consideration
Thermogram was interpreted and analyzed to obtain information about the percentage weight loss at different temperatures which gives information about sample composition, product formed after heating and kinetic parameters. Thermodynamic and kinetic parameters have been evaluated using Sharp-Wentworth and Freeman-Carroll methods as follows
1) Freeman-Carroll method
The following expression was given by Freeman-Carroll [8] to evaluate activation energy (Ea) and order of reaction (n) for the decomposition of copolymer
?log?(dW/dt)?logWr = n – Ea2.303R
= n – Ea2.303R .?(1/T)?logWr
.?(1/T)?logWr ……………………………(1)
……………………………(1)
Where, dW/dt is the rate of change of weight with time; Wr = Wc - W, where, Wc is the weight loss at completion of reaction and W is the fraction of weight loss at time t; Ea is the energy of activation; n is the order of reaction, T and R is the temperature and gas constant respectively.
The plot between the terms ?log?(dW/dt)?logWr versus ?(1/T)?logWr
versus ?(1/T)?logWr gives a straight line. The slope of the line -Ea/2.303R, gives energy of activation (Ea) and intercept on Y-axis as order of reaction (n) where x=0.The change in entropy (ΔS), frequency factor (Z), apparent entropy change (S*) can also be calculated by further calculations.
gives a straight line. The slope of the line -Ea/2.303R, gives energy of activation (Ea) and intercept on Y-axis as order of reaction (n) where x=0.The change in entropy (ΔS), frequency factor (Z), apparent entropy change (S*) can also be calculated by further calculations.
2) Sharp–Wentworth method
The equation derived by Sharp and Wentworth [9] has been used to determine activation energy, free energy change and entropy change for the copolymer decomposition
logdC/dT1-C = log(A/β) –Ea2.303R
= log(A/β) –Ea2.303R .1T
.1T …………….………………..(2)
…………….………………..(2)
Where, dC/dT is the rate of change of fraction of weight with change in temperature and β is the linear heating rate dT/dt.
III. RESULTS AND DISCUSSION
The synthesized 2,4-DHP-4-PAF-III terpolymer resin was found to be soluble in solvents such as DMF, DMSO and THF while insoluble in almost all other organic solvents. The yield of the terpolymer resin was found to be 78%.
A. Elemental Analysis
The 2,4-DHP-4-PAF-III has been analyzed for carbon, hydrogen and nitrogen content. The empirical formula and empirical formula weight has been assigned by the result of elemental analysis which is presented in Table-1.The percentage of element determined by elemental analysis is found good agreement with the calculated values.
Table-1. Elemental analysis and empirical formula of terpolymer resin
|
Terpolymer resin |
% of Carbon observed calculated |
% of Hydrogen observed calculated |
% of Nitrogen observed calculated |
Empirical formula of repeat unit |
Empirical formula weight |
|
2,4-DHP-4-PAF-III |
66.47 67.50 |
6.17 5.62 |
3.80 4.37 |
C36H36N2O9 |
640 |
B. FT-IR Spectra
Infrared spectra of 2,4-DHP-4-PAF-III terpolymer resin is presented in Figure-2. A broad and strong band appeared in the region 3291 cm-1 may be due to the stretching vibrations of phenolic hydroxyl (Ar-OH) group exhibiting intramolecular hydrogen bonding. The presence of -NH in pyridyl moiety may be ascribed to sharp and weak band at 2939 cm-1. The sharp band displayed at 1628 cm-1 may be due to the stretching vibrations of Ar-CO- carbonyl group .The bands obtained at 1373 cm-1 indicates the presence of methylene bridges in the terpolymer chain. A sharp band appearing at 1495 cm-1 describes the presence of >C=C< (aromatic) group [10-12].
(aromatic) group [10-12].
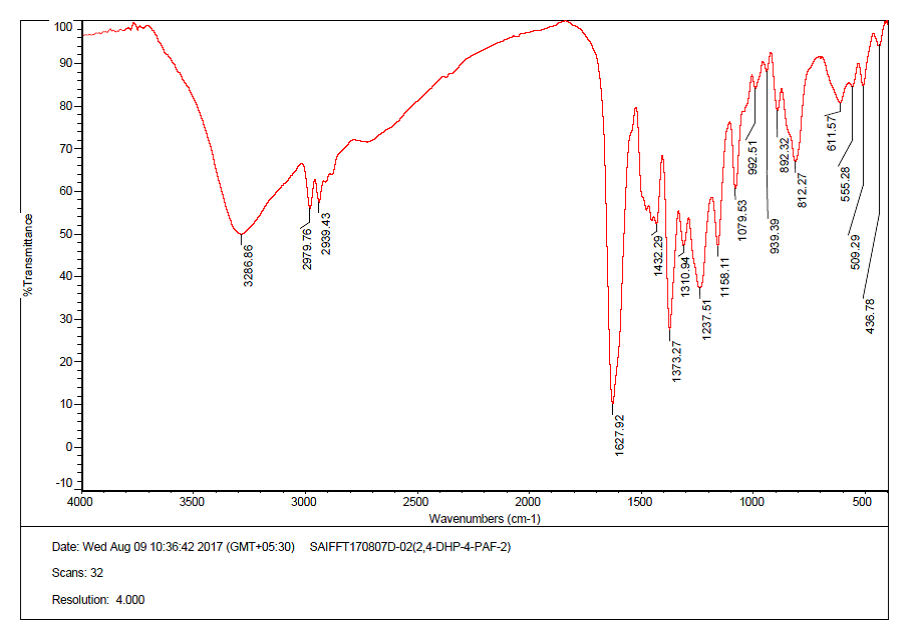
Fig. 2: FT-IR Spectra of 2, 4-DHP-4-PAF-III terpolymer resin
C. 1H- NMR Spectra
The 1H NMR spectra of 2, 4-DHP-4-PAF-III terpolymer resin was scanned in DMSO-d6 and the spectra is depicted in Figure-3. The doublet signal observed at 2.6 (δ) ppm may be attributed to the methylene proton of Ar-CH2-bridge. The triplet signal in the region 7.6 (δ) ppm indicates the presence of the amido proton Ar-CH2–NH. The weak multiplet signal (unsymmetrical pattern) in the region at 7.1 (δ) ppm may be due to the aromatic proton (Ar-H). The singlet signals in the range at 6.3 (δ) ppm may be due to phenolic hydroxyl proton. The much downfield chemical shift for phenolic -OH group indicate clearly the intramolecular hydrogen bonding on –OH group. A peak appeared in the region 1.2 (δ) ppm indicates the methyl proton of Ar-CO-CH2-CH3 group [13-15].
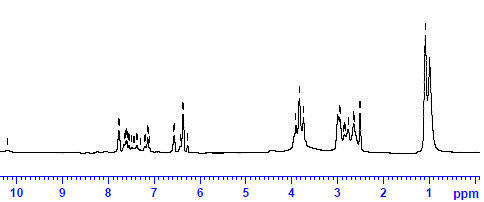
Fig.3: NMR Spectra of 2, 4-DHP-4-PAF-III terpolymer resin
D. Thermogravimetry
The Terpolymer 2,4-DHP-4-PAF-III has been subjected to thermo gravimetric analysis and data used to assess the degradation pattern. The thermal decomposition behavior curve for the resin is depicted in Figure-4, which exhibits four stage decomposition and its ranges are incorporated in the Table-2. The first stage decomposition was slow and ranged between 40-215 0C corresponds to mass loss of 2.77 % found and 52.73 % calculated which may have been due to entrapped water molecule. The second stage decomposition starts from 215 0C to 435 0C, corresponding the gradual mass loss of 44.20 % found and 44.22 % calculated which represents the degradation of hydroxyl group and –COCH2CH3 group attached to aromatic benzene ring. The third stage decomposition at 435-603 0C, corresponding to 83.82 % rapid mass loss of aromatic nucleus with methylene group against calculated 83.89 % . The fourth stage starts from 603 0C to 640 0C corresponding to removal of pyridylamine moiety with observed mass loss of 99.96% against calculated 100%. The half decomposition temperature for 2, 4-DHP-4-PAF-III copolymer is found to be 4900C [16, 17].
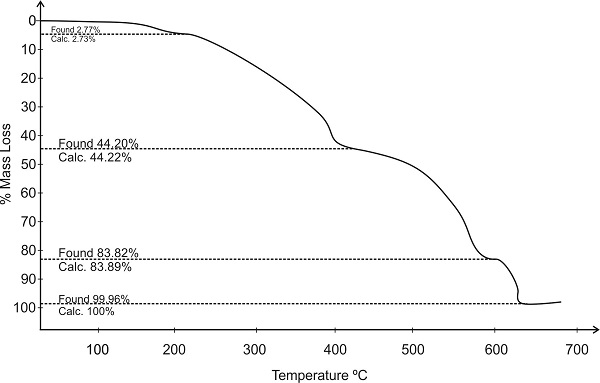
Fig. 4: Decomposition Pattern of 2, 4-DHP-4-PAF-III Terpolymer Resin
Table-2. Thermal degradation behavior of 2, 4-DHP-4-PAF-III terpolymer resin
|
Terpolymer resin |
Stages of dec-omposition |
Temperature range (0C) |
Group degraded |
% Weight loss |
|
|
Observed |
Calculated |
||||
|
2,4-DHP-4-PAF-III |
First |
40 – 215 |
Loss of water molecule |
2.77 |
2.73 |
|
Second |
215 - 435 |
–OH and –CO-CH2-CH3 groups |
4.20 |
4.22 |
|
|
Third |
435 – 603 |
benzene ring and –CH2 group |
83.82 |
83.89 |
|
|
Fourth |
603 – 640 |
Pyridine moiety |
99.96 |
100 |
|
E. Thermoanalytical Data
A plot of percentage mass loss versus temperature is depicted in Figure-4 for a representative 2,4-DHP-1,5-DANF-III terpolymer. The method described by Sharp-Wentworth and Freeman-Carroll were adopted to obtain the relative thermal stability of copolymer. The thermal stability of copolymer, based on the initial decomposition temperature has also been used here to define their relative thermal stability, neglecting the degree of decomposition. A representative thermal activation energy plot of Sharp-Wentworth method (Figure-5) and Freeman-Carroll method (Figure 6, 7) for the copolymer have been shown. By using thermal decomposition data and then applying above methods the activation energy (Ea) is calculated which are not perfectly in agreement with each other. But the average Ea calculated by Sharp-Wentworth and Freeman-Carroll methods are nearly same. The thermodynamic parameters have been calculated on the basis of thermal activation energy and by using equations in (3),(4),(6) and (7). These values are incorporated in the Table-3 [18,19].
1) Entropy change
Intercept = log[KRh∅Ea ] +?S2.303R
] +?S2.303R …………………………………………………………………(3)
…………………………………………………………………(3)
where,
K= 1.3806 × 10-16 erg/deg/mol,
R= 1.987 cal/deg/mol, h=6.625 × 10-27 erg sec, ?= 0.166,
?S= change in entropy, Ea= activation energy
2) Free energy change
?F= ?H - T?S …………………………………………………………………………………….(4)
where,
?H= activation energy
T = temperature in kelvin
?S= entropy change from equation (3)
3) frequency factor
B2/3= log?(ZEa)R∅ ………………………………………………………………………………………..(5)
………………………………………………………………………………………..(5)
B2/3= log(3) + log [1- 31-α ] – logp(x) …………………………………………………………..(6)
] – logp(x) …………………………………………………………..(6)
where,
Z= Frequency factor,
B= Calculated from (6)
log p(x)= calculated from Doyle table corresponding to activation energy
4) Apparent entropy change
S*= 2.303R log ZhRT* ………………………………………………………………………………..(7)
………………………………………………………………………………..(7)
where, T*= Temperature at which half of the compound decomposed from its total loss
Table-3: Results of Thermogravimetric Analysis of 2, 4-DHP-4-PAF-III Terpolymer Resin
|
Terpolymer resin |
Half decomposition temperature (T*),0C |
Activation energy (Ea), KJ/mol |
Entropy change (?S), (J) |
Free energy change (?F),(J) |
Frequency factor(Z) (sec.-1) |
Apparent entropy (S*),(KJ) |
Order of reaction (n) |
|
|
FC |
SW |
|||||||
|
2,4-DHP-4- PAF-III |
490 |
29.47 |
28.81 |
-225.14 |
200.59 |
446.7 |
-656.94 |
0.80 |
FC= Freeman-Carroll SW= Sharp-Wentworth
The graphs obtained by Freeman–Carroll and Sharp-Wentworth methods are fairly good straight line or linear by ignoring some abnormal points. This indicates the decomposition reaction of copolymer does not obey the first order kinetics perfectly. Due to abnormally low value of frequency factor [Z] it may be classified as slow reaction. The slow reaction is also supported by the negative values of entropy change. The value of entropy change (?S) also indicates that the activated polymer has more ordered structure than the reactants [20, 21].
Conclusion
The terpolymer 2,4-DHP-4-PAF-III was prepared by condensation reaction of 2,4-dihydroxypropiophenone, 4-pyridylamine with formaldehyde in the presence of acid catalyst. The proposed structure of terpolymer has been determined from the elemental analysis, FT-IR and 1H-NMR spectral studies. Low values of collision frequency factor (Z) may be concluded that the decomposition reaction of synthesized terpolymer can be classified as ‘slow reaction. In TGA, the energy of activation evaluated from the Sharp-Wentworth and Freeman-Carroll methods are found to be nearly equal and the kinetic parameters obtained from Freeman-Carroll method are found to similar, indicating the common reaction mode. The decomposition reaction was started at higher temperature, indicating a terpolymer 2, 4-DHP-4-PAF-III is thermally stable at higher temperature.
References
[1] Kapse S. K., Hiwase V. V., Kalambe A. B., and Kene J. D., Comparative thermokinetics study of terpolymeric resins derived from p-hydroxyacetophenone, resorcinol and glycerol, Research Journal of Chemical Sciences, 4(2), (2014). 81-86. [2] Kamlakar. A. Nandekara, Jivan. R. Dontulwara and Wasudeo. B. Gurnule, Thermal Behaviour of Newly Synthesized Copolymer Derived from Salicylic acid, and Thiosemicarbazide, Der Pharma Chemica, 4(4), (2012), 1644-1652. [3] Dharkar K. P., Khamborkar A. K. and Kalambe A. B., Thermal Degradation Analysis of Melamine-Aniline-Formaldehyde Terpolymeric Ligand, Research Journal of Chemical Sciences, 2(12), (2012), 11-16. [4] Wasudeo B. Gurnule, Yashpal U. Rathod, Amoli D. Belsare , Narayan C. Das, Thermal degradation and antibacterial Study of transition metal complexes derived from novel terpolymer ligand, Materials Today: Proceedings, 29, (2020), 1044–1049. [5] Priyanka U. Belsare and Anil B. Zade, Synthesis, Characterization and Thermogravimetric Analysis of 2, 2’-Biphenol Based Terpolymer Resins , Chem Sci Trans., 2(4),( 2013, 1136-1147. [6] Pawan P Kalbende , Mangesh V Tarase and Anil B Zade, Synthesis, characterization and non-isothermal thermo-gravimetric analysis of cross-linked phenol based copolymer resins, Research Journal of Pharmaceutical, Biological and Chemical Sciences, RJPBCS, 3(4), (2012), 1276-1294, [7] R. Srinivasulu, S. Madhusudan, M. Ashok Kumar , V. Nikil Murthy , N. Kartikeyan, Synthesis and Characterization of Polymer Nanocomposites Filled with Nanoclay/Aerosil on Mechanical and Thermal Properties, International Journal of Nanomaterials and Biostructures, 3(4), (2023), 51-56. [8] E. S. Freeman and B. J. Carroll, The application of thermo analytical technique to reaction kinetics, the thermogravi- Metric evaluation of the kinetics of decomposition of calcium oxalate monohydrate, Phys. Chem., 62(4), (1958), 394 –397. [9] J. B. Sharp and S. A. Wentworth, Kinetic analysis of themogravimetric, Anal. Chem., 41(14), (1969), 2060-2062. [10] N. C. Das and W. B. Gurnule, Metal Ion Uptake Properties of Chelating Ion Exchange Copolymer Synthesized from 2, 4- Dihydroxypropiophenone and 4-Pyridylamine, International Journal for Research in Applied Science & Engineering Technology, 12(8), (2024), 1163-1169, 2024. [11] S. S. Rahangdale, N. C. Das, K. S. Vajpai and W. B. Gurnule, Synthesis, characterization and thermal degradation study of copolymer resin-II: resulting from 2-hydroxy, 4-methoxybenzophenone, 1, 5- diaminonaphthalene and formaldehyde, I J R B A T, 8(1), 2020, 194-204. [12] S. K. Mandavgade, J. R. Dontulwar, and W. B. Gurnule, Thermal degradation study of new polymer derived from 8-hydroxyquinoline 5-sulphonic acid and catechol, Der Pharma Chemica, 4(4), (2012), 1695–1703. [13] N. C. Das and W. B. Gurnule , Removal of heavy metal ions from water using chelating copolymer resin-iv derived from 2-hydroxy, 4-methoxybenzophenone, 1, 5-diaminonaphthalene and formaldehyde, International Journal of Science and Research Archive, 2024, 12(01), 3058–3065. [14] N. C. Das , K. S. Vajpai , A. P. Ganorkar and W. B. Gurnule, Synthesis, Characterization and Morphology of OrganicCopolymer Resin-III Resulting from 1,5- Diaminonaphthalene, 2,4- Dihydroxypropiophenone and Formaldehyde, International Journal of Advanced Research in Science, Communication and Technology, 12( 4), (2021), 322-328. [15] N. C. Das and W. B. Gurnule, Electrical Conductivity Study of Novel Organic Copolymer Resin Synthesized from 2-Hydroxy, 4- Methoxybenzophenone, 1, 5-Diaminonaphthalene and Formaldehyde, International Journal of Advanced Research in Science, Communication and Technology, 4(1), (2024), 520-525. [16] M. V. Tarase, A. B. Zade and W. B.Gurnule, Kinetics of thermal degradation studies of some new terpolymers derived From 2, 4-dihydroxypropiophenone, oxamide, and formaldehyde, Journal of Applied Polymer Science, 116(2), (2010), 619–627. [17] A. N. Gupta, V. V.Hiwase and A. V. Kalambe, Thermal degradation and kinetic study of terpolymer resin-Iderived from p-hydroxybenzaldehyde, succinic acid and ethylene glycol, Der Pharmacia Lettre, 5 (2), (2013), 105-112. [18] W. B. Gurnule and S. P. Dhote, Synthesis, characterization and thermal degradation studies of copolymer resin derivedfrom 4-hydroxybezophenone, melamine and formaldehyde, J. Chem. Pharm. Res.,5(12), (2013), 942-949 [19] D. T. Masram, K. P. Kariya and N. S. Bhave, thermal degradation and electrical conductivity measurementstudy of resin derived from salicylic acid, hexamethylenediamine and formaldehyde, Elixir Appl. Chem., 48, (2012), 9557-9562. [20] S. S. Butoliya, W. B. Gurnule, and A. B. Zade, Study of non-isothermal decomposition and kinetic analysis of 2,4-dihydroxybenzoic acid-melamine-formaldehyde copolymer, E-Journal of Chemistry, 7( 3), (2010), 1101- 1107. [21] R. N. Singru, A. B. Zade and W. B. Gurnule, Thermoanalytical study and kinetics of new 8-hydroxyquinoline-5-sulphonic acid-oxamide-formaldehyde copolymer resin, E-Journal of Chemistry, 6(S1), (2009), 171-182.
Copyright
Copyright © 2024 N. C. Das, W. B. Gurnule. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64546
Publish Date : 2024-10-11
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

